Corrosion Chemical Or Physical Change
Table of Contents
-
- What is a Physical Modify?
- What is a Chemical Change?
- Rusting of Iron
- Crystallization
- Ofttimes Asked Questions – FAQs
What is a Concrete Modify?
Properties such equally shape, size, state of the substance and its colour are known as physical properties. Physical properties are afflicted past the physical change.
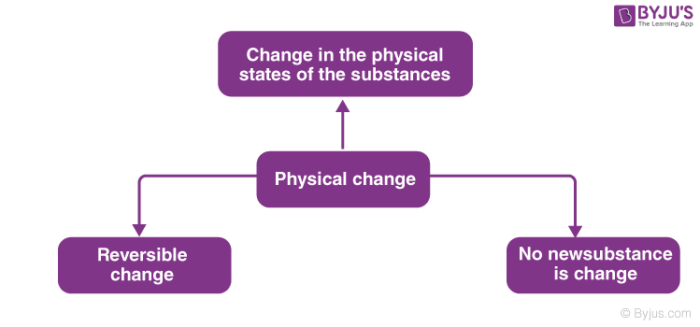
For Instance, when ice changes to water, there is a modify in the concrete state of ice, i.due east. it changes from solid to liquid. If we cool the water again, ice will exist formed making it a reversible reaction. Also, no new substance is formed in the above physical change.

What is a Chemical Change?
A change in which one or more new substances are formed is known as chemic change.
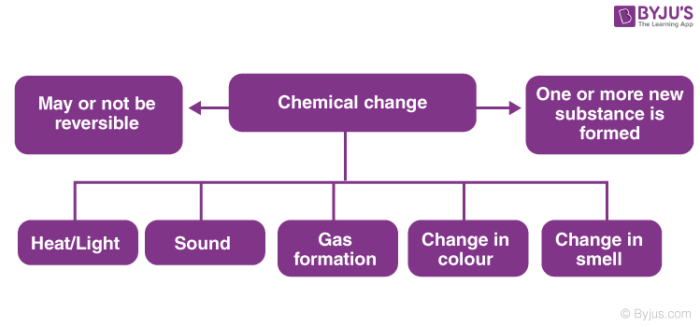
For Example, when the iron is exposed to air and moisture, rust formation takes place. Rust is nothing but Atomic number 26 Oxide; a new substance formed out of the reaction. The colour of the surface of the iron also changes. Hence, rusting of atomic number 26 is a chemical change.

Rusting of Iron
When substances fabricated of iron are exposed to oxygen and moisture (water), rusting takes place. Rusting removes a layer of material from the surface and makes the substance weak. Rusting is a chemic change.
Chemic reaction taking identify during rusting is shown below.
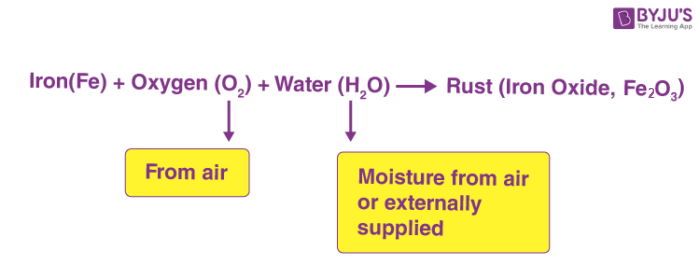
Rusting is undesirable and methods are used to avoid rusting.

The process of depositing zinc on the iron to forestall rusting is known as galvanization. A striking example of the employ of galvanization is the h2o pipes used in houses.
Crystallization
A physical process of obtaining large crystals of a pure substance from its solution is known as crystallization. Crystallization is a physical modify.

Frequently Asked Questions – FAQs
What are physical and chemical changes?
A chemical transition is the effect of a chemic reaction, and a physical change occurs where the construction of affair changes simply not the chemic identity. Examples of chemical transformations include burn down, frying, rusting, and rotting. Examples of physical changes are to simmer and freeze.
What defines a chemical change?
Chemical reactions requiring the rearrangement of atoms of one or more compounds and the modification of their chemical properties or structure resulting in the creation of at least one new substance: iron rust is a chemical alteration.
Is Melting zinc a chemical modify?
A chemic reaction is a mechanism that happens by converting ane or more compounds into one or more than other compounds. No chemical reaction is registered. However, if the mixture absorbs free energy in the course of heat, the zinc may react chemically with the sulphur to form the compound zinc sulphide (ZnS).
Which process is a chemic change?
Textile modifications arise as a substance becomes a new material, called chemical synthesis or, similarly, chemical decomposition into two or three distinct compounds, combined with another. These mechanisms are called chemic reactions, and they are unremarkably not reversible or past boosted chemical reactions.
What is the importance of chemical alter?
Chemic processes allow ane to understand matter'due south backdrop. We can larn its chemical properties by observing the way a sample interacts with another matter. These backdrop may be used to classify an unknown specimen or to predict how different kinds of matter may react with each other.
Corrosion Chemical Or Physical Change,
Source: https://byjus.com/chemistry/physical-change/
Posted by: myerstoop1998.blogspot.com



0 Response to "Corrosion Chemical Or Physical Change"
Post a Comment